Hepa-Merz®
Profile
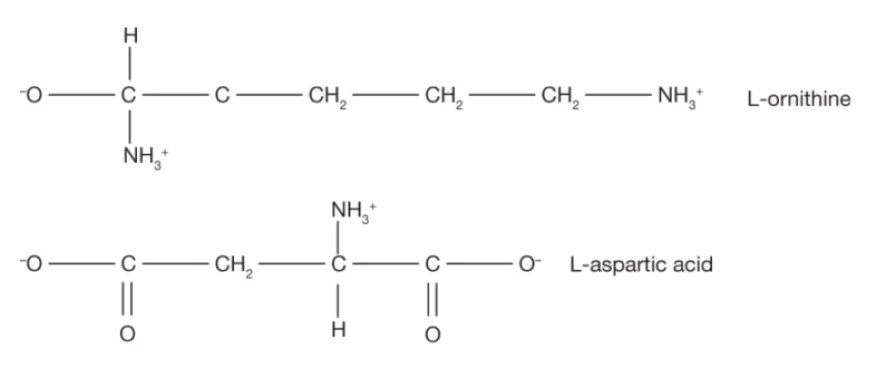
Hepa-Merz® is a stable salt of the naturally-occurring amino acids, L-ornithine and L-aspartic acid (LOLA). LOLA increases ammonia detoxification in two ways:
- Liver (periportal hepatocytes): activation of the urea cycle in the liver by making available the metabolic substrate ornithine which activates the enzyme carbamoyl phosphate synthetase in the cirrhotic liver1
- Brain, muscle, liver (perivenous cells), the substrates ornithine and aspartate promote the formation of glutamate which results in stimulation of ammonia detoxification via glutamine synthesis1
L-ornithine-L-aspartate (LOLA)
Pharmacokinetics1
LOLA is rapidly absorbed and metabolised, it has bioavailability of 82.2% ± 28% after either intravenous or oral administration. Orally administered LOLA dissociates into its component amino acids, which absorbed in the small intestine by active transport. Elimination half-life is short at approximately only 40 minutes with some L-aspartate appearing unchanged in the urine.
Formulations of Hepa-Merz®1
LOLA is available in several pharmaceutical forms (and are different in different countries and regions of the world – please check your local prescribing information):
- Granules: Hepa-Merz®1 sachet contains 3 g of LOLA
- Infusion concentrate: 10 ml concentrate contain 5 g LOLA in water for injection
Hepa-Merz® indications
The Hepa-Merz® registration may differ in different countries and regions of the world – please check your local prescribing information for the precise indications.
Hepa-Merz® is indicated for the treatment of disorders that accompany, or are secondary to hepatic detoxification impairment (e.g. liver cirrhosis) with symptoms of latent and manifest hepatic encephalopathy. Infusion formulation is specifically indicated for use in cases of precoma or coma.1
Hepa-Merz® is contraindicated in patients with severely impaired renal function (serum creatinine level of over 3 mg/ 100 ml can be regarded as a guideline).1
1. Hepa-Merz® Summary of Product Characteristics. Revision Date 09/2016