Hepa-Merz®
Safety and tolerability
Data on safety and tolerability of Hepa-Merz® from observations of therapeutic use, clinical studies and especially placebo-controlled double-blind trials found that the tolerability of LOLA is very good with no reported cases of serious adverse drug reactions.1
The use of LOLA in general practice led to reports of spontaneous suspected adverse drug reactions including occasional nausea, rarely vomiting (only with infusion therapy especially with a high infusion rate). Symptoms were generally transient and reversible after dose reduction or by lowering of the infusion rate.
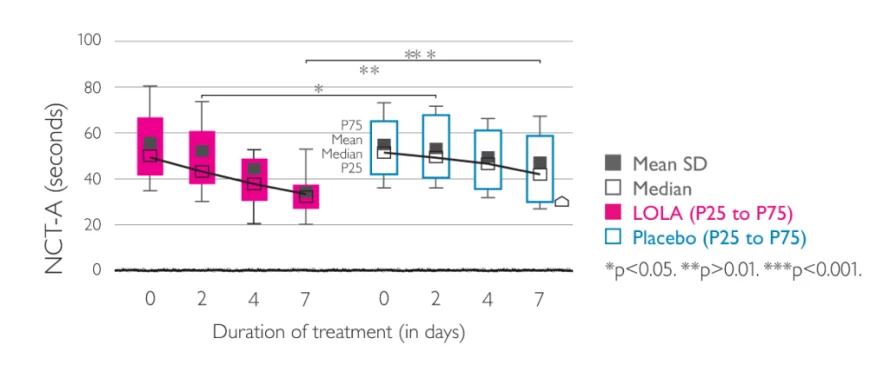
A multicentre, randomised, double-blind, placebo-controlled trial was carried out to demonstrate the efficacy and tolerability of LOLA infusion. This trial showed efficacy of LOLA in comparison with placebo with respect to the mental state, the ammonia concentration, and the performance time for number connection test-A (NCT-A). The product was generally well tolerated, causing no serious adverse reactions.2
LOLA is well tolerated in HE
Effects of LOLA (left) vs placebo (right) on the time required to perform NCT-A. Figures given are the mean (black square) and standard deviation, median (white square), 25th and 75th percentiles, and the group differences between the two treatments.2
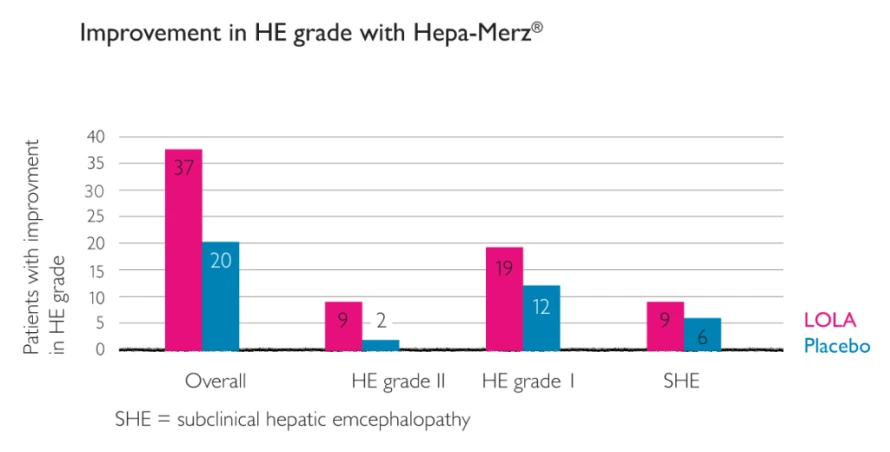
Number of patients with improvement in HE grade after seven days’ treatment with LOLA or placebo in the various subgroups.2
- Observations of Hepa-Merz® in therapeutic use, clinical studies, and placebo-controlled double-blind trials prove that the tolerability of LOLA is very good. No cases of serious adverse drug reactions have been recorded.1
- In placebo-controlled, double-blind trials with large case numbers, there were three cases (5% of LOLA patients) of mild gastrointestinal disturbances, i.e. nausea/vomiting, with infusion therapy;2 there were no cases in the placebo group. In the largest placebo-controlled trial with LOLA granules, there were no adverse events in either group.3
- LOLA therapy is generally very well tolerated and adverse reactions rarely occur, usually in the form of mild gastrointestinal disturbances. To prevent such reactions, a maximum infusion rate of 5 g LOLA infusion concentrate per hour is recommended.1
- Consistent with its mechanism of action, LOLA leads to the increased formation of urea, which has to be eliminated via the kidneys. LOLA should, therefore, not be used in cases where there is severe impairment of renal function. As a general rule, the creatinine level should not be greater than 3 mg/dL.1
LOLA therapy is generally very well tolerated and adverse reactions may occur rarely, in the form of mild gastrointestinal disturbances. To prevent such reactions, a maximum infusion rate of 5 g LOLA infusion concentrate per hour is recommended.1
1. Liver Disease and Hepatic Encephalopathy Scientific Product Monograph, 2012 Merz Pharmaceuticals.
2. Kircheis G et al. Therapeutic efficacy of L-ornithine-L-aspartate infusions in patients with cirrhosis and hepatic encephalopathy: results of a placebo-controlled, double-blind study. Hepatology. 1997;25(6):1351-60.
3. Stauch S et al. Oral l-ornithine-l-aspartate therapy of chronic hepatic encephalopathy: results of a placebo-controlled double-blind study. J Hepatol. 1998;28:856-964.