Hepatic encephalopathy
Classification and clinical features of hepatic encephalopathy
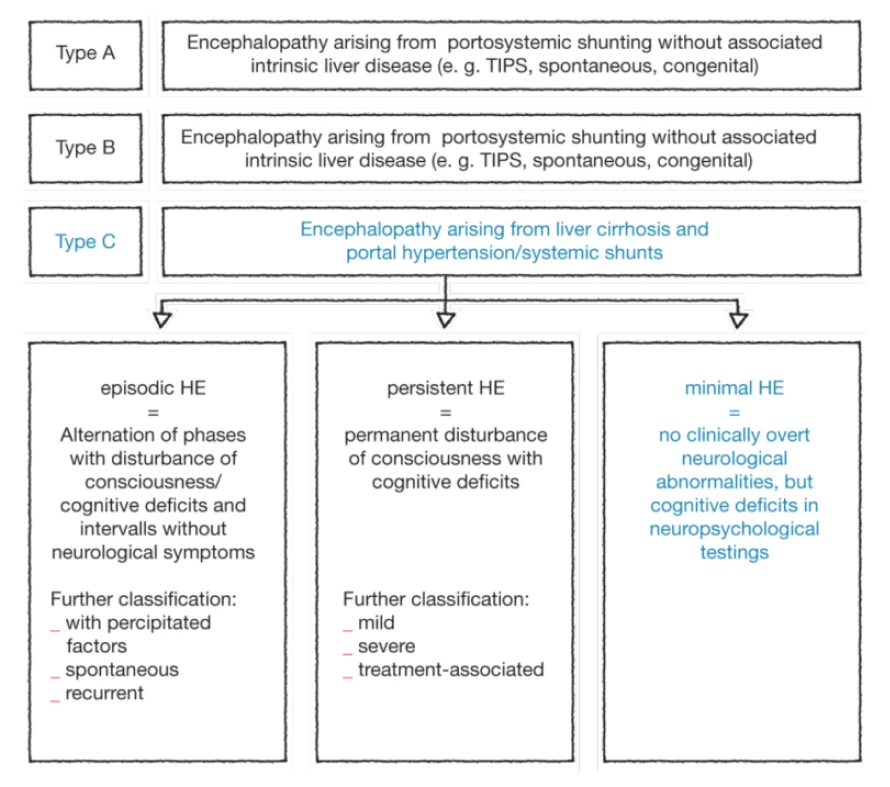
Please notify the fact that the West Haven Criteria were established but then adapted several times focussing more and more on the clinical symptoms of the patients. The criteria were adapted by the ISHEN with a complete new approach of classification: the “poverty-covert-model”.
The classification of HE1,2
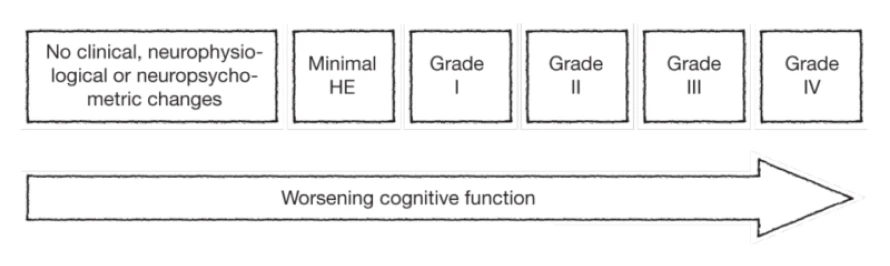
Overt hepatic encephalopathy (HE) is traditionally classified into four grades according to the West Haven criteria. These widely used criteria classifies HE into 5 grades (ranging from minimal [grade 0] to coma [grade 4] based on the patient’s level of consciousness, intellectual function and behaviour, as detailed in the table below. Stage 0 corresponds to the subtype of HE called minimal hepatic encephalopathy (MHE), which is highly prevalent (22-74%) among patients with liver dysfunction. MHE is defined as HE without grossly evident neurologic abnormalities, but with cognitive deficits that can be revealed by psychometric testing.1,3
West-Haven grading of HE1,3
Semi-quantitative staging of mental status in HE according to the West Haven criteria (modified from Conn et al.3).
Grade | Symptoms |
0 (minimal) | Normal examination; if impaired psychometric test; minimal HE |
1 | Mild lack of awareness Shortened attention span Impaired performance of addition / subtraction Mild asterixis or tremor |
2 | Lethargy Disorientation Inappropriate behaviour Obvious asterixis; slurred speech |
3 | Somnolence but responsive to stimuli Gross disorientation; bizarre behaviour Muscular rigidity and clonus; hyper-reflexia |
4 | Coma (unresponsive to verbal or noxious stimuli) Decerebrate posturing |
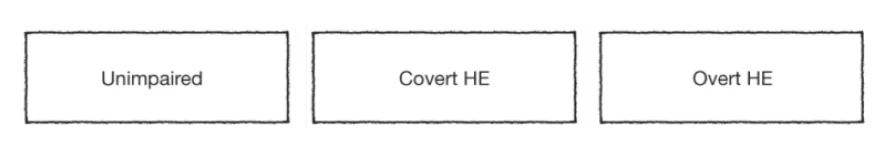
The West-Haven criteria have been adapted several times based on an increasing focus on clinical symptoms. Furthermore, diagnostic differentiation between MHE and low-grade, overt HE (i.e., WH I) can be clinically difficult.4
The following new classification was therefore proposed by ISHEN, the International Society for Hepatic Encephalopathy and Nitrogen Metabolism:4
Minimal hepatic encephalopathy (MHE)
MHE, the mildest form of HE, is characterized by subtle motor and cognitive deficits, and impairs health-related quality of life (HRQOL). Cirrhotic patients with MHE have a normal neurological and mental status by the standards of clinical examination, yet demonstrate quantifiable neuropsychological defects. The term MHE refers to the subtle changes in cognitive function, electrophysiological parameters, cerebral neurochemical/neurotransmitter homeostasis, cerebral blood flow, metabolism, and fluid homeostasis that can be observed in patients with cirrhosis who have no clinical evidence of hepatic encephalopathy. These subtle neurocognitive abnormalities primarily affect attention, speed of information processing, and motor abilities and coordination that are not recognizable on standard neurological examination. These neurocognitive abnormalities are independent of sleep dysfunction or problems with overall intelligence. It has been well-described that MHE has a subtle but negative impact on a patient’s spatial skills, motor skills, the ability to perform complex tasks such as driving, and even quality of life. MHE predicts the development of overt HE and is associated with poor survival.5-7
Symptoms of MHE
MHE adversely affects HRQOL. Cognitive impairment in MHE mainly affects complex activities involving attention, information, processing and psychomotor skills such as driving a car, planning a trip, etc. whereas basic activities of daily life, such as shopping, dressing, personal hygiene, etc. are preserved. Patients with MHE had a significant impairment of daily functioning, such as social interaction, alertness, emotional behavior, sleep, work, home management, recreation and pastimes compared with cirrhotic patients who did not have MHE. Treatment with lactulose improved both cognitive functions and HRQOL; improvement in the latter was linked to improvement in cognitive function.5-7
Symptoms of HE
Common symptoms presented by a patient with HE include tiredness, weakness, fatigue, exhaustion, lack of motivation. Symptoms intensify during the day, especially after physical or mental activities. In the advanced stage of HE there are mental changes including:1
- Impaired concentration
- Poor memory
- Disturbance of the sleep-wake cycle
- Mood swings
- Irritability
Signs and symptoms of HE: symptoms commonly reported by patients suffering from HE and signs for healthcare professionals to look out for in diagnosing HE
Patients with HE report these Symptoms:1
- lack of appetite with aversion to certain foods
- disturbances of the sense of taste
- nausea
- diarrhoea
- pain in the right upper abdomen
- increase in abdominal circumference (ascites)
- weight loss
- pruritus
- asterixis (also called “liver flap“), a short loss in muscle tone followed by a fast corrective reflex
- fever
- sexual disorders (in men)
Healthcare professionals should look for these symptoms:1
- abdominal wall vascular collaterals (caput medusa)
- clubbing and hypertrophic osteoarthropathy
- Cruveilhier-Baumgarten murmur – a venous hum in portal hypertension
- Gynaecomastia
- testicular atrophy
- hepatomegaly
- splenomegaly
- jaundice, scleral icterus
- Kayser-Fleischer ring – a brown-green ring of copper deposit around the cornea, pathognomonic for Wilson´s disease
- fetor hepaticus – a sweet, pungent breath odour
1. Ferenci P et al. Hepatic encephalopathy–definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35(3):716-21.
2. Zhan T, Stremmel W. The diagnosis and treatment of minimal hepatic encephalopathy. Dtsch Arztebl Int. 2012;109(10):180-7.
3. Conn HO et al. Comparison of lactulose and neomycin in the treatment of chronic portal-systemic encephalopathy. A double blind controlled trial. Gastroenterology. 1977;72(4 Pt 1):573-83.
4. Bajaj JS et al. Review article: the design of clinical trials in hepatic encephalopathy–an International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) consensus statement. Aliment Pharmacol Ther. 2011;33(7):739-47.
5. Dhiman RK, Chawla YK. Minimal hepatic encephalopathy. Indian J Gastroenterol 2009;28:5-16.
6. Prasad S, Dhiman RK, Duseja A, Chawla YK, Sharma A, Agarwal R. Lactulose improves cognitive functions and health-related quality of life in patients with cirrhosis who have minimal hepatic encephalopathy. Hepatology 2007;45:549-59.
7. Amodio P, Montagnese S, Gatta A , Morgan MY. Characteristics of minimal hepatic encephalopathy. Metabolic Brain Disease 2004; 19:253-267.